
When catheterizing, you may experience urine flow stops. This can happen even though your bladder is not completely empty.3

Do you ever wonder if your bladder is fully emptied after using a catheter? Have you found yourself repositioning the catheter to make sure no urine is left behind? Or do you worry about the number of UTIs you have every year?
Get ready for the latest innovation in catheter technology for women! Coloplast's Micro-hole Zone Technology is here to set a new standard for bladder emptying1 and reduce the risk of UTIs.2


When catheterizing, you may experience urine flow stops. This can happen even though your bladder is not completely empty.3
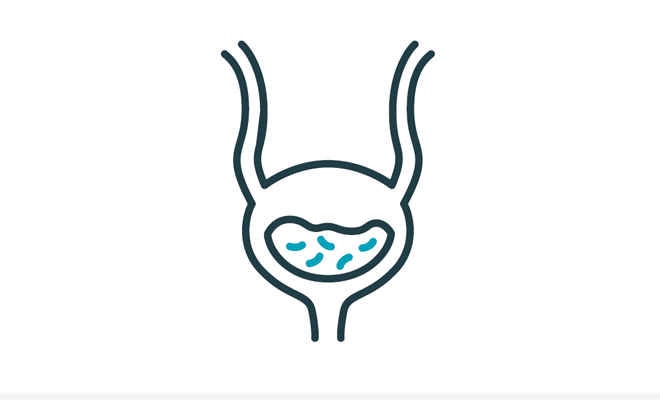
If urine is left in the bladder after catheterization, bacteria in the urine and bladder may quickly multiply4 and could potentially cause a UTI.3
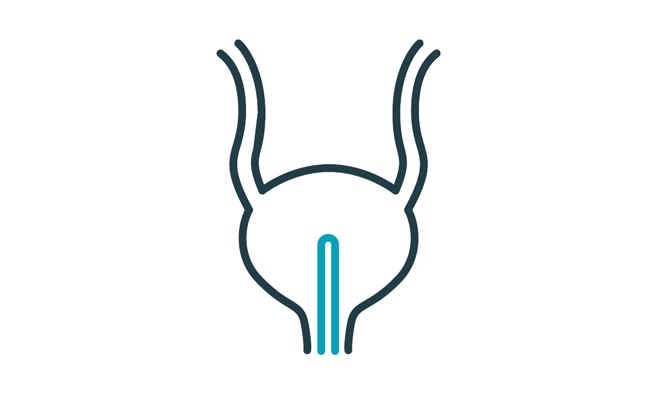
Adjusting the catheter until the bladder is completely empty is important to help reduce your risk of UTIs3 – but with the next generation female intermittent catheter from Coloplast, you are able to avoid having to adjust the catheter.1

Most conventional catheters have two eyelets and require you to adjust the catheter to empty your bladder.
The new Micro Hole Zone Technology by Coloplast is designed to completely drain urine from your bladder without having to readjust or repositon the catheter.1
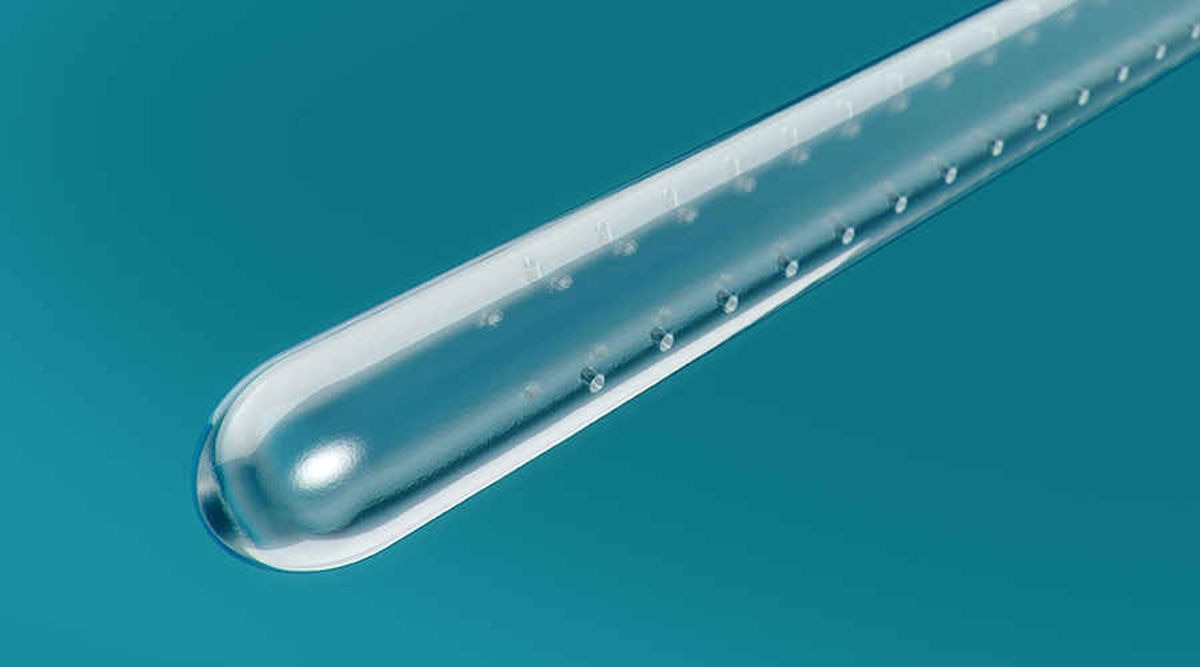
With its new micro-hole zone, our upcoming female intermittent catheter addresses more catheter-related UTI risk factors2 than ever before.
With recyclable product container material6, it is purposefully designed with the user and environment in mind.

Many factors can raise your risk of contracting a UTI.2 Understanding the different factors can help you take measures to avoid UTIs. Be informed with our UTI risk factor quick guide.
Prior to use, refer to product labeling for complete product instructions for use, contraindications, warnings and precautions.
Luja female is indicated for use by patients with urine retention and patients with a post void residual volume (PVR) due to neurogenic and non-neurogenic voiding dysfunction. The catheter is inserted into the urethra to reach the bladder allowing urine to drain. The product is indicated for female patients only (adults and children of and above the age of 2 years). Available by prescription only. Patients performing self-catheterization should follow the advice of, and direct questions about use of the product to, their medical professional. Apply with caution if the patient produces urine with many particles clearly distinguishable by the naked eye, as it may lead to transient urine retention. Before using the device, carefully read the product labels and information accompanying the device including the instructions for use which contain additional safety information. For single-use only; discard it after use. If you experience symptoms of a urinary tract infection, or are unable to pass the catheter into the bladder, contact your healthcare professional. The risk information provided here is not comprehensive. To learn more, talk to your healthcare provider. For further information, call Coloplast Corp. at 1-866-226-6362 and/or consult the company website www.coloplast.us.